Abstract
Background: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, potentially life-threatening hematologic disease characterized by various degrees of hemolysis, bone marrow failure, and thrombophilia. Pegcetacoplan (PEG), a C3 complement-inhibitor approved by the FDA for treatment of adults with PNH, has demonstrated improved hemoglobin (Hb) levels for PNH patients with screening Hb levels <10.5 g/dL and prior suboptimal response to eculizumab (ECU; C5-inhibitor) (Hillmen P, et al., N Engl J Med, 2021 384 (11):1028-1037) or complement-inhibitor naïve PNH patients (Wong RS, et al., Blood, 2020 136 [Supplement 1]). While these studies have demonstrated positive results for patients with lower baseline Hb levels, the efficacy and safety of PEG in PNH patients with baseline Hb ≥10.0 g/dL has not been evaluated.
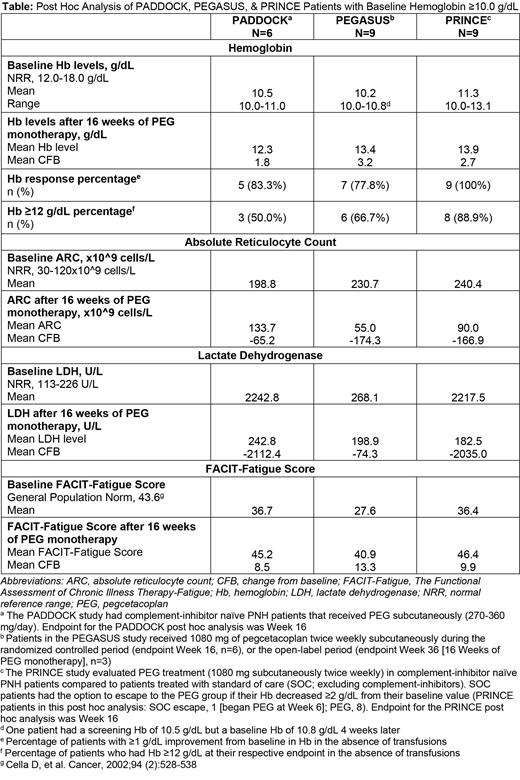
Aims: This post hoc analysis evaluated the safety and efficacy of 16 weeks of PEG therapy for PNH treatment in a subgroup of patients with baseline Hb levels ≥10.0 g/dL from the PADDOCK (NCT02588833) Phase 1b, PEGASUS (NCT03500549) Phase 3, and PRINCE (NCT04085601) Phase 3 studies.
Methods: Adult PNH patients from the PADDOCK, PEGASUS, and PRINCE studies with baseline Hb levels ≥10.0 g/dL and no transfusions within 14 days of baseline were included in this analysis. PADDOCK evaluated PEG therapy at 270-360 mg/day subcutaneously (SC) in complement-inhibitor naïve PNH patients. PEGASUS enrolled PNH patients that remained anemic despite stable ECU treatment (≥3 months) with Hb levels <10.5 g/dL at the screening visit: Patients were randomized 1:1 to ECU or PEG (1080 mg SC twice weekly) during the randomized controlled period (RCP) through Week 16, after which ECU patients were switched to PEG monotherapy during the open-label period (OLP) through Week 48. PRINCE compared PEG treatment (1080 mg SC twice weekly) in complement-naïve patients to patients receiving standard of care (SOC; excluding complement-inhibitors). SOC patients had the option to escape to the PEG group if Hb decreased ≥2 g/dL from baseline levels.
Post hoc analyses were performed at Week 16 for PADDOCK and PRINCE, Week 16 for PEGASUS patients treated with PEG during the RCP, and Week 36 for PEGASUS patients who switched from ECU to PEG during the OLP (16 weeks of PEG monotherapy). Endpoints included change from baseline (CFB) in Hb, percentage of patients with Hb response (≥1 g/dL Hb increase without transfusion), percentage of patients with Hb ≥12 g/dL without transfusion, and CFB in absolute reticulocyte count (ARC), lactate dehydrogenase level (LDH), and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) after 16 weeks of PEG monotherapy. Transfusion recipients (PADDOCK, n=0; PEGASUS, n=2, [1 RCP, 1 OLP]; PRINCE=0) were defined as Hb non-responders and censored for all analyses.
Results: In total, 24 patients had baseline Hb levels ≥10.0 g/dL at the baseline visit: six from PADDOCK, nine from PEGASUS (6 RCP, 3 OLP), and nine from PRINCE (1 SOC escape [began PEG at Week 6], 8 PEG). Overall, patients from all three studies achieved improved Hb levels (Table) with the majority demonstrating a Hb response and Hb ≥12 g/dL (Table). Mean decreases in ARC and LDH (Table) were observed with mean LDH less than the upper limit of normal at 16 weeks in all studies (Table). Clinically significant increases in mean FACIT-Fatigue scores were observed in all three groups (Table). No thrombotic incidents were noted in this patient population. Two PEGASUS patients had transfusions of 5 units over 2 days and of 6 units over 16 weeks due to breakthrough hemolysis.
Conclusions: These results demonstrate that PEG can further improve hematological outcomes in PNH patients with baseline Hb levels ≥10.0 g/dL who are complement-inhibitor naïve or remained anemic after ECU therapy. While these patients had near normal Hb at baseline, they also had elevated ARC prior to PEG therapy, suggesting ongoing hemolysis. Improvements in mean Hb level, Hb response, ARC, LDH level, and FACIT-Fatigue score suggest that PEG is effective in this subgroup resulting in a decline in overall hemolytic activity and a clinically significant improvement in fatigue. Overall, these results suggest that the utility of PEG is not restricted to naïve and complement-inhibitor experienced PNH patients with low baseline Hb but can be efficacious in those with near-normal baseline Hb, resulting in further clinical improvements in relevant PNH parameters.
Panse: Apellis Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Grunenthal: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Speakers Bureau; Chugai: Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Peffault De Latour: Apellis Pharmaceuticals Inc: Consultancy, Honoraria; Alexion: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Swedish Orphan Biovitrum AB: Consultancy, Honoraria. Schafhausen: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Swedish Orphan Biovitrum AB: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees. Straetmans: Alexion: Membership on an entity's Board of Directors or advisory committees. Al-Adhami: Apellis Pharmaceuticals: Current Employment. Ajayi: Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Chen: Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Yeh: Apellis Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Wong: Alexion: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis Pharmaceuticals: Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal